Sclerotinia (White Mold) Disease on Carrots
Sclerotinia disease on carrots is becoming a serious economic disease in many vegetable growing areas. It is considered to be the most destructive disease of stored carrots. Significant losses can occur during the long storage over the winter. Under the right conditions for the pathogen, the losses can be as high as 50% due to sclerotinia rot and secondary bacterial soft rot infections.
The fungal pathogen, Sclerotinia sclerotiorum, has a very wide host range, including canola, sunflower, soybeans, beans and a number of vegetable crops. It appears that increasing acres of susceptible crops may be correlated with higher disease levels in carrots. Sclerotia of the fungus overwinter in soil and crop residue.
Disease Symptoms
In the field the early signs of the disease would be a water soaked lesion at the base of the foliage, and if humidity is high, the infection could be accompanied with cottony white mycelial growth on the carrots and crop residue. In the storage, the white mycelium of the pathogen grows rapidly and infects carrots over large areas.
Figure 1: Field infection of carrots can occur directly by the mycelia (filaments) growing within a dense crop canopy or by ascospores falling on a wet canopy.

Figure 2: During harvest, lightly infected carrots cannot be separated out; they get collected and put into storage along with healthy carrots. Damage to tops can hinder mechanical harvest.

Figure 3: During storage, Sclerotinia rot disintegrates the carrots, and also leads to secondary bacterial rotting.

Disease Cycle
The disease is favoured by high soil moisture and a dense canopy. In summer with warm and moist soils within a crop canopy, the sclerotia germinate and produce apothecia. These apothecia produce millions of microscopic ascospores, which get disseminated over long distances with wind and rains, especially by thunder storms. Ascospores landing on wet leaves can lead to infection. Foliage infection is often followed by crown infection. Many of the sclerotia germinate directly producing mycelial structures which can infect the crop residue, carrot leaves and crowns. Under favourable conditions the leaves may turn brown and die-off. Damage to foliage tops prevent mechanical harvest of such carrots. A few weeks after infection black sclerotial bodies, normally 5-15 mm in size, develop on old infections. These infected carrots may often not show any significant symptoms by the time of harvest and may be missed during grading for long term storage.
Field warm carrots brought into the storage building are cooled down as soon as possible and held for long term storage under conditions of high humidity. The optimum temperature for disease development ranges from 13-18oC, but temperatures above 0oC will allow the disease to progress in storage if there is free moisture on carrots and RH >92%.
In storage the disease development starts from infections initiated in the field, from un-cleaned pallet boxes or storage walls. High humidity in the storage allows the white mycelium of the pathogen to grow rapidly and infect carrots over large areas. During storage many of the infected carrots develop sclerotia bodies, and are diagnostic. Infected spots in the carrot pile with poor air circulation could develop a secondary infection by soft rot bacteria and lead to greater storage losses.
Disease Management
- Cultural methods include crop rotation with non-host crops. Improving air movement within the crop canopy can prevent conditions favourable for disease development. Air movement within a crop can be reduced by effective weed control, increasing row-row spacing, growing on ridges or raised beds and/or cutting excess foliage between rows using mechanical cutters. In wet growing seasons or periods of frequent rains, the chopped foliage residue may cover the ground and provide favourable conditions for mycelial development and growth on the residue.
Figure 4a: Figure 4b: 

Figures 4a and 4b: showing the increased gap between pairs of rows to increase air movement within the carrot canopy. - Chemical fungicides registered for sclerotinia control are limited in numbers. There may be a few fungicides which have activity against sclerotinia, but are registered for other disease(s) than sclerotinia. For example, Lance is registered for Alternaria leaf spot control but not sclerotinia disease in carrot. Scholar (fludioxonil) has a post-harvest treatment Emergency Use Registration only (until end of 2011).
- Biofungicide CONTANS has been registered for management of sclerotinia disease in carrots. The fungicide is a suspension of fungal spores of Coniothyrium minitans, a selection of a natural soil-borne fungus. The spores, exclusively infect the sclerotia of pathogen Sclerotinia sclerotiorum and prevent them from germinating directly into mycelium or producing apothecia. The bio-fungicide, thus reduces the overall inoculum source in the fields; unlike the chemical fungicides which only prevent infection of the crop and have negligible activity on the inoculum source. A lower dosage of the fungicide can be used after a susceptible crop has been harvested or in the fall prior to planting a susceptible crop; it can also be applied early in the spring before planting a susceptible crop – thus there is flexibility in timing and rate of application. The fungal spores need about 3 months of exposure to sclerotia for good activity.
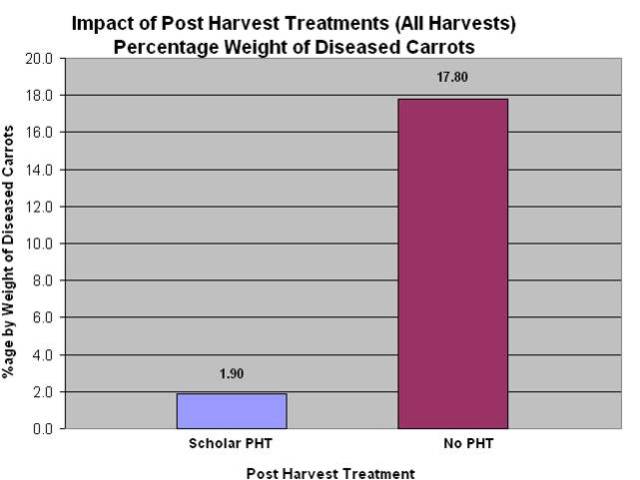
- Vikram Bisht, Plant Pathologist and Tom Gonsalves, Vegetable Specialist, MAFRD along with a commercial carrot grower, conducted a 2-year (2010 and 2011) field research trial to show the efficacy of CONTANS and Scholar on management of sclerotinia in carrots. Data from the 2010 trials showed that CONTANS treatment was also effective in reducing the levels of storage rot due to sclerotina compared to the untreated control. In this trial, Serenade (Bacillus subtilis, QST 713 strain) was not effective. Data also shows that Scholar is very effective as post-harvest spray on carrots or as dipping treatment, in controlling the levels of storage rot as compared to the untreated controls. Data for 2011 has yet to be completely analysed.
Figure 5: CONTANS treatments were effective in reducing sclerotinia diseased carrots, 2010.
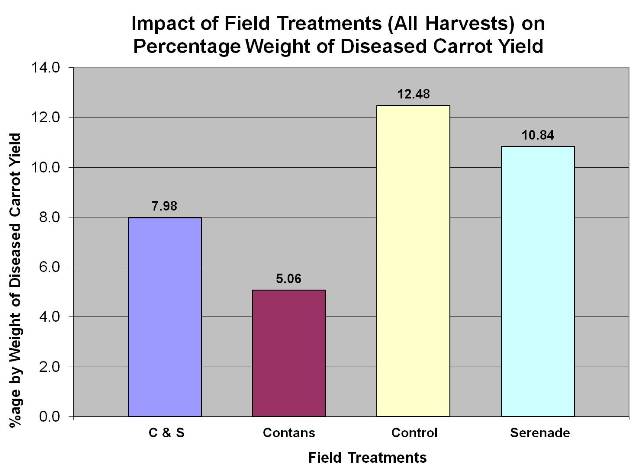
Figure 6: Post-harvest spray or dip treatment of carrots dramatically reduced storage loss due to sclerotinia disease. There was no difference between spray or dip treatments.