Clubroot Of Brassica Crops
Importance | Symptoms | Pathogen | Management | Soil Testing
Summary/Key Points
- Clubroot is a disease of brassica crops (most notably cabbage, cauliflower, broccoli, rutabaga, and canola). It can substantially reduce seed quality and oil content in canola, resulting in economic losses.
- The chief concern to Manitoba producers is the longevity of the club root pathogen (Plasmodiophora brassicae) in soil. It has the potential to survive for ten to twenty years in the absence of a canola crop. Once the pathogen has established in a field, it is difficult to manage. The years between successive canola crops in a rotation would have to be increased from existing practices.
- Clubroot has been recognized as a problem on brassica vegetable crops in ON, QC, BC, and Atlantic Canada for several years.
- In 2003, the first report of clubroot on canola in western Canada was made from fields near Edmonton, AB. As of December 2007, clubroot had been reported in 11 municipalities in Alberta, including one in southern Alberta.
- Alberta has put together an action plan to manage the clubroot situation to prevent clubroot outbreaks outside the 11 municipalities in Alberta.
- There are two types of clubroot spores a short-lived "swimming phase" (zoospore) and a long-lived resting spore, which is the chief concern.
- Clubroot resting spores can be spread on contaminated plant material such as transplants, any seed with "earthtag" (soil adhering to a surface); and contaminated soil on equipment (e.g. truck tires)
- Currently there is no means of dealing with clubroot once observed in canola. The only preventive actions include longer crop rotations, and disinfesting contaminated equipment to prevent spread of soilborne resting spores.
- Typically the clubroot pathogen prefers acidic soils which are not common in Manitoba. There are a number of cases where there have been outbreaks in soil pH levels comparable to those of Manitoba soils. The level of risk to the canola crop is uncertain in Manitoba, but is still a concern that requires attention.
- P. brassicae, has been reported in Manitoba on symptomatic cruciferous vegetables. Reports have been very sparse, with the first note dating back to an unconfirmed report on rutabaga in 1925. In the 1980s, clubroot was found on market garden cruciferous vegetables. The most recently known detection of clubroot in Manitoba occurred in 2005, on canola. The symptoms were of very low severity. Surveys of canola in Manitoba ( not targeted at clubroot) have not detected plants with symptoms of clubroot, or soilborne resting spores in canola production areas.
- To prevent the introduction of clubroot spores to a new field, attempts should be made to prevent the movement of soil from one area to another, by means of soil carried on equipment, personnel, planting material, etc.
- Crop rotations away from brassica crops, of at least 4 years will be necessary to reduce the population of spores in a field. Shorter rotations will favour the development of clubroot.
- No feasible chemical controls exist for the management of clubroot on Canola.
- Brassica weeds should be managed to eliminate a potential reservoir of the pathogen that could contribute to future outbreaks.
- Soil and plant testing for clubroot is conducted by commercial laboratories, but it is recommended that fields be scouted and identification of other potential problems be conducted first. Submitting a suspected sample to the Manitoba Crop Diagnostic Centre is recommended.
Importance
Clubroot, caused by the pathogen Plasmodiophora brassicae, is a serious disease of Brassica crops in most production areas around the world, having caused serious losses to vegetable crops such as cabbage, rutabaga, radish, cauliflower, broccoli, and Brussels sprouts. While considered strictly a disease of brassica (crucifers) it is known that P. brassicae can cause disease on other plant families (See Table 1). The role of many of these plants in the cycle of the disease is not well understood and the practical importance may be very limited.
Table 1: Non brassica plants on the Canadian prairies susceptible to infection by P. brassicae.
| Latin Name | Common Name |
|---|---|
| Agrostis stolonifera | Creeping bent grass |
| Dactylis glomerata | Orchard grass |
| Fragaria spp. | Strawberry |
| Lolium perenne | Perennial rye grass |
| Papaver rhoeas | Corn Poppy |
| Rumex spp. | Dock |
Clubroot has been present in Canada for years, and is not a new problem. British Columbia, Ontario, Quebec, New Brunswick, Nova Scotia, Prince Edward Island Newfoundland, and very recently Alberta, have all been identified as areas where clubroot has been observed and well established.
Manitoba has reported clubroot in the past, but this disease had typically appeared to have a very limited impact on crop production. The first report of clubroot in Manitoba occurred in the late 1920s on Rutabaga, followed by anecdotal observations of the disease in Market Garden vegetables in the mid 1980s. In 2005, clubroot symptoms of low severity were observed at an incidence level of about 0.4%.
In the United States, there have been a number of reports of clubroot on brassica crops from several states, varying in degrees of severity and incidence.
In the two states bordering Manitoba, there have been sporadic clubroot observations on rutabaga (Brassica napus L. var napobrassica) from both Minnesota and North Dakota and field mustard (Brassica rapa) in Minnesota. Clubroot is not a common occurrence in either state as reports date back to the 1950s. There have been numerous observations from a number of states, on a range of brassica crops, primarily vegetables, and weeds in the brassica family.

Figure 1: Clubroot symptoms on young canola roots. Courtesy of the Western Committee on Plant Disease Digital Image Collection, original photo by I.R. Evans.
Recent Developments
In 2003, clubroot was reported on canola (Brassica napus) for the first time in Canada, in areas near Edmonton, AB. In the four years subsequent to the initial findings, the disease has been detected in 250 fields, with incidence levels ranging from below 30% (low) to above 70% (high). There are concerns that the soilborne pathogen could be spread to other canola production areas in Alberta, Saskatchewan, and Manitoba, causing significant yield losses.
Symptoms
The above-ground symptoms of clubroot resemble most root diseases, with wilt symptoms suggesting the plant is under water and nutrient stress. Plants may appear to have a blue-green colour; this is more apparent in leafy vegetable crops. Pale green to yellowish leaves may be observed primarily on the lower leaves initially, and later in the season plants may show mid-day wilting symptoms. Pre-mature ripening may also result, leading to reduced yield and crop quality, (this is not solely a clubroot symptom but, if clubroot is suspected, this would be a good reason to look for galls).
When a plant is pulled from the soil, the most obvious symptom is the clubbed appearance of the roots. Individual roots may have spherical to elongated swollen portions often club-shaped. In the initial stages of plant development, the galls are firm and white inside; later, they become brown and decompose. When removed from the ground for closer inspection, the stem may detach from the club, leaving the easily broken clubbed roots in the soil.
Extensive galling of the roots can disrupt the uninfected roots as well. The degree of severity of the disease varies with the growth stage of the plant, root morphology, and the length of time that the plant is subjected to infections by the pathogen.
Infection of young plants by P. brassicae typically results in the greatest damage, as young tissues can be penetrated directly. As the plant develops, the older thickened roots and underground stems require wounding for P. brassicae to gain entry into the host plant.
The overall impact of clubroot is a progressive stunting and wilting of the aboveground parts of the plant. This decline can be exacerbated further as the physical damage to the root caused by the fast growing and abnormally enlarging cells of the diseased tissues prevent the formation of a cork layer. This results in compromised roots that are more susceptible to secondary and weakly pathogenic organisms.

Figure 2: Clubroot symptoms on young cabbage roots. Courtesy of the Western Committee on Plant Disease Digital Image Collection, original photo by I.R. Evans.
Clubroot "Mimic" Symptoms
Hybridization nodules, are small rounded structures that may be present at root nodes. While not common, these structures may be observed on the roots of canola plants. These have nothing to do with clubroot, but may be misdiagnosed as clubroot galls. To distinguish these from clubroot symptoms, examine the interior texture. A clubroot gall is spongy or marbled, hybridization nodules appear as healthy roots, of uniform texture and colour. Hybridization nodules do not decompose as clubroot galls do.
Herbicide damage which may cause thickening of roots may also be misidentified as clubroot galls.
Pathogen Biology
Plasmodiophora brassicae Woronin is the causal agent of clubroot. It is a soilborne obligate parasite that requires living root tissue of the host to complete its life cycle. This pathogen is considered a plasmodiophoromycete or endoparasitic slime mold. These organisms have much more in common with protozoan animals than the true fungi such as Sclerotinia sclerotiorium (white mold) or Phoma lingam (blackleg).
The chief concern related to clubroot is the durability and lifespan of the resting spores. The resting spores may remain capable of initiating new infections for as long as twenty years. Thus, once a field becomes infested with a population of spores, it will remain infested for a long period of time.
The disease cycle is shown in Figure 3.
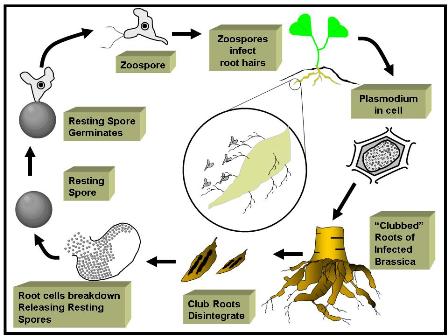
Figure 3: Disease cycle of clubroot caused by Plasmodiophora brassicae.
Within the plant roots, P. brassicae exists as a plasmodium (comparable to an amoeba). As roots develop, the plasmodium gives rise to zoosporangia or resting spores which, upon germination, produce zoospores. They may infect other healthy root hairs via movement through a saturated soil environment.
The single zoospore produced from the resting spores forms a cyst on a root. The cell contents are injected into root hairs and the development of a plasmodium begins. Within a few days, the plasmodium splits into cells within a zoosporangium, containing four to eight secondary zoospores.
The new zoospores are released into the soil through perforations in the cell wall of the host plant. These zoospores may initiate new infections and the process begins again, with the formation of more plasmodia. A new group of secondary zoospores is produced and they subsequently enter the soil environment.
As the plasmodia moves though host tissues, they become established in some cells. The result is abnormal divisions and an increase from the normal cell size.
Generally not all cells in diseased tissues are colonized by a plasmodium, but non- invaded cells of diseased tissues are also stimulated to grow abnormally.
The plasmodium-infected clubs not only utilize much of the food required for the normal growth of the plant, but they also interfere with the absorption and translocation of mineral nutrients and water through the root system. This stress on the plant affects quality and yield.

Figure 4: Clubroot symptoms on young cabbage plants (right) compared to healthy roots on the left. Note the differences in plant sizes. Photo Courtesy of the Western Committee on Plant Disease Digital Image Collection, original photograph by R. Howard.
Clubroot Management
Preventative Measures
Scouting of canola fields for clubroot (and other diseases) is an approach that cannot be overstated. Look for plants that are wilting, reduced in size and may be ripening early. Then determine the causes. The Crop Diagnostic Centre in Winnipeg is an excellent resource to identify canola diseases. Plants can also be sent for testing to 20/20 seed labs in Alberta.
P. brassicae is soilborne and therefore any practice that will move infested soil is a potential risk for introduction to new areas. Observations from canola fields in Alberta have suggested the highest concentration of spores are at entry points into the field.
Equipment, tools, vehicles, containers, and personnel should have all mud and soil removed prior to entry into another field. While removal of soil can be a laborious process, movement of soil is the most likely means by which clubroot resting spores will disperse (See Figure 5).
The role of soil on planting material (that includes non-brassica crops, and even crops not susceptible to clubroot) should be considered. Earth tag (or soil tag)(soil adhering to a surface) on seeds/propagating material could be a potential risk. For example: potato seed pieces are large and have a potential to bring more adhering soil to a field. There could be a risk of introducing resting spores to a clean field, if infested potatoes are planted. The degree of risk from earth tagged seeds/tubers is unclear, but producers should be aware there is a potential concern.
Use of common untreated seed, may also present a risk due to the increased likelihood of earth tag.
The resting spores of clubroot, can survive passage through the digestive tract of livestock. Spreading of manure, from livestock fed with infested crop debris, could enable resting spores to be spread to areas free of P. brassicae spores.
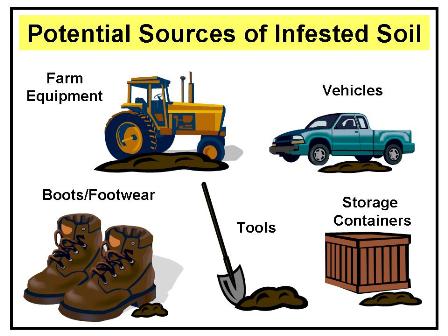
Figure 5: The potential means by which clubroot infested soil could be spread from one production area to another.
Management After Establishment
Once a field has become infested with clubroot, eradication is not feasible. The approach is to minimize the development of P. brassicae to prevent high concentrations of spores from being produced.
Soil conditions have a significant impact on clubroot symptom severity. Saturated soil at a temperature of about 20-24°C and acidic soil increases the severity of the disease. Above a pH of 7.2, spore germination is usually inhibited but disease has been reported to develop.
Rotation
Once a field becomes infested, there is really only one option, crop rotations of at least four years, though five to seven years or longer have been recommended, depending on the spore concentration.
Clubroot is reported to have a "half-life" of four years which implies that every four years, 50% of the existing spores will be unviable. After eight years 50% of the remaining spores present be unviable (meaning that after 8 years 25% of the initial resting spores are still viable). The risk of the disease in a field after the absence of a susceptible crop will depend on the levels of the spores (spore load) after the last brassica crop. In fields that are heavily infested with clubroot, the time between brassica crops may have to be lengthened from the four year (three year between brassica crops).
In order for disease to occur, a pathogen, a susceptible host, and suitable environmental conditions are required. While the best approach to managing any disease is to avoid the introduction of the pathogen to an area to begin with, once established however, eradication (elimination of the pathogen), can be quite difficult in practice. This is clearly the case with clubroot. The presence of one spore in a field is not generally of great concern, but it is the dose or concentration of resting spores that one needs to be concerned with.
Levels below 1000 spores /gram have been considered low for disease development. It is the basis of this threshold which is the rationale for management through crop rotations. There is variability in the resting spores which influences the longevity of the population. As the period of time away from a suitable host increases, the population/concentration of viable spores decreases.
The rate at which the concentration (spore load) decreases is influenced by soil type, soil pH and the level of spore concentration prior to removal of a suitable host. The higher the concentration/infestation, the longer it takes before a brassica crop could be put in with a reduced probability of disease development.
Zoospores require free water to move within the soil. Heavy soils and poorly drained sand soils, are conducive to disease development. Avoid compaction of soils as this could favour the development of clubroot.
Soil conservation practices such as direct seeding which reduce soil movement via wind or water erosion, as well as prevention of soil movement on tillage equipment, will be of benefit for clubroot management. Excessive moisture due to flooding or pooling of water within a field can increase the risk of clubroot if the pathogen is present. In these situations, neutral to alkaline soils (pH 7.0-7.2) which are typically less suitable for clubroot development may still be at risk for clubroot development.
Weed Management:
There are a number of weeds (see Table 2) that can serve as a host for clubroot and continue the production of new resting spores. Elimination of these weeds when present, is critical for reduction of disease risk in infested fields.
Table 2: Brassica weed hosts of Plasmodiophora brassicae on the Canadian Prairies. (There are no reports from the prairie provinces of these weeds having symptoms of clubroot, but the disease has been reported on these weeds in other parts of North America).
| Latin Name | Common Name |
|---|---|
| Armoracia rusticana | Horseradish, red cole |
| Brassica hirta | White mustard |
| Brassica kaber | Wild Mustard |
| Camelina sativa | False Flax |
| Camelina microcarpa | Small Seeded False Flax |
| Capsella bursa-pastoris | Shepherd's Purse |
| Erysimum asperum | Western Wallflower |
| Lepidium campestre | Pepperwort |
| Rorippa islandica | Marsh cress |
| Rorippa sylvestris | Creeping yellow cress, yellow field cress |
| Sisymbrium altissimum | Tumbling Mustard |
| Sisymbrium officinale | Hedge Mustard |
| Thlaspi arvense | Stinkweed |
Chemical Control-Fungicides:
There are fungicides registered for clubroot control which are only practical and economical for use on Brassica vegetable crops (Table 3). These products are not registered for canola. They are all the same active ingredient -pentachloronitrobenzene (quintozene).
Table 3: Fungicides (all group 14) registered for use on vegetable crops for management of clubroot (Plasmodiophora brassicae).
| Product | PCP# | Crops |
|---|---|---|
| Quintozene (Terraclor®) 75% | 07251 | Broccoli, brussel sprouts, cabbage, and cauliflower |
| Adobe 75WP | 28663 | Broccoli, brussel sprouts, cabbage, and cauliflower |
| Crusoe 75 WP | 28238 | Broccoli, brussel sprouts, cabbage, and cauliflower |
| Quintozene 75WP | 27416 | Broccoli, brussel sprouts, cabbage, and cauliflower |
| Quintozene 75WP | 11425 | Cole Crops |
Chemical Control-Soil Liming
Application of lime to soil to increase the pH, in order to make the soil less suitable for clubroot development has been tried. It does not eradicate existing populations. This has been attempted in vegetable production, but this has not proven to be completely reliable. In fields with high concentrations of spores, liming has not provided acceptable results.
It would not be practical at this time to attempt liming to a pH of 7.3 -7.5 as a method of clubroot control in canola.
Summary of Management Practices (as suggested in Alberta's Clubroot Management Plan)
- Use long rotations between canola crops (one canola crop every four years) to prevent a severe infestation from developing.
- Control volunteer canola and cruciferous weeds.
- Practice good sanitation by restricting movement of potentially contaminated soil to non-contaminated regions.
- Use direct seeding and other soil conservation practices to reduce soil movement via wind or water erosion, as well as soil movement on tillage equipment.
- Scout canola fields regularly and carefully. Identify causes of wilting, stunting, yellowing and premature ripening.
- Avoid use of straw, hay or greenfeed, silage and manure from infested or suspicious areas.
- Avoid common untreated seed (including canola, cereals and pulses). Make sure seed is free of earth tag (the effect of seed treatments is unknown).
Soil Testing And Plant Testing (taken from 20/20 Seed Labs)
A recently developed test using molecular biology techniques has been commercialized. At present 20/20 Seed Labs offers the service for $90.00 a soil sample (as of April 29, 2008). The procedure for collecting samples is below. For further information please go to the 20/20 Seed lab website.
Collecting Soil Samples:
- Survey results have shown that the highest incidence of clubroot occurs at the entrance to fields.
- Sample in a W-shaped pattern at entranceways out to a maximum 150 feet into the field. Low-lying points within the field, homestead garden sites, and soil clumps that may have fallen off machinery are also hot-spots for possible pathogen presence.
- DO NOT sample randomly across the field.
- Clear all loose organic matter from the soil surface and collect the top 5-10 cm of the A-horizon, or less as the depth allows, without taking any of the B-horizon.
- Submit a minimum 2 Cup sample of soil. Air dry and send in a Ziploc bag.
Suspect Plants:
- Infected plants are concentrated sources of pathogen, and represent a significant escalation in the amount of inoculum present in a field.
- Scout for suspect plants (planted or volunteer) and submit fresh, frozen or dried roots for testing.
Suggested Websites For Further Clubroot Information:
- Alberta: Clubroot Disease of Canola and Mustard
- Alberta: Clubroot Management Plan
- Canola Council information on clubroot
References:
- Agrios, G.N. 1997. Plant Pathology. Academic Press, San Diego, 635 pp.
- Anonymous. 1960. Index of Plant Diseases in the United States, U.S. Department of Agriculture Handbook. No. 165. Washington, D.C. 531 pp.
- Conners, I.L. 1967. An annotated index of plant diseases in Canada and fungi recorded on plants in Alaska, Canada, and Greenland. Queens Printer, Ottawa 381 pp.
- Donald, E.C., Porter, L.J., Faggian, R. and Lancaster, R.A. 2006. An integrated approach to the control of clubroot in vegetable brassica crops. Acta Hort. (ISHS) 706:283-300
- Farr, D.F., Bills, G.F., Chamuris, G.P., and Rossman, A.Y. 1995. Fungi on Plants and Plant Products in the United States APS Press, St. Paul. 1252 pp.
- Ginns, J.H. 1986. Compendium of Plant Disease and Decay Fungi in Canada.Minister of Supply and Services Canada. Ottawa 416pp.
- Kendrick, B. 1992. The Fifth Kingdom. Mycologue Publications Waterloo, ON. 406 pp.
- Looman, J. and Best, K.F. 1987. Budd's Flora of the Canadian Prairie Provinces. Minister of Supply and Services Canada. 863 pp.
- Murukami, H., Tsushima, S., and Shishido, Y. 2002. Factors affecting the pattern of the dose response curve of clubroot disease caused by Plasmodiophora brassicae. 48: 421-427.
- Preston, D.A. and Dosdall, L. 1955. Minnesota Plant Diseases, USDA Plant Disease Epidemics and Identification Section. Special Publication no.8. 184pp.
- Rimmer, S.R., Shattuck, V.I., and Buchwaldt, L. 2007. Compendium of Brassica Diseases. APS Press, St. Paul, 117 pp.
- Strelkov, S.E., Tewari, J.P., and Smith-Degenhardt, E. 2006. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can. J. Plant Pathol. 28:467-474.
- Tewari, J.P., Strelkov, S. E., Orchard, D., Hartman, M., Lange, R., and Turkington, T.K. 2005. Identification of clubroot of crucifers on canola (Brassica napus) in Alberta. Can. J. Plant Pathol. 27:143-144.
- Tremblay, N., Bélec, C., Lawrence, H., and Carisse, O. 1999. Clubroot of crucifers control strategies. Agriculture and Agri-Food Canada. Saint-Jean-sur Richelieu, PQ. 3 pp.
For further information, contact an Manitoba Agriculture/ MASC Service Centre

